Translate this page into:
Giant apocrine carcinoma of the breast: A case report with review
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Invasive apocrine carcinoma of the breast is rare. The criteria for the diagnosis are uncertain with the variable reported incidence in the literature. The use of androgen receptor studies by immunohistochemistry and its potential response to androgen analogs necessitates the subtyping of this tumor. We report a case of giant invasive apocrine carcinoma of the breast involving the entire breast parenchyma without skin and chest wall infiltration. The clinical, pathological, immunohistochemical, and ultrastructural findings are discussed with emphasis on morphological and immunohistochemical features that aid in the diagnosis of this rare tumor.
Keywords
Androgen receptor
apocrine carcinoma
breast
electron microscopy
oncocytic carcinoma
Introduction
Apocrine carcinoma is one of the rare subtypes, which accounts for about 0.5%–4% of invasive breast malignancies. It has a similar presentation, imaging features, and growth pattern as invasive carcinoma, no special type.1 Apocrine carcinoma is defined by the presence of apocrine differentiation in at least 90% of tumor cells.2 We present one such case with emphasis on morphology, immunohistochemistry, and ultrastructural features that help in the diagnosis.
Case Report
An elderly female presented with a history of right breast lump for 2 years and recent sudden increase in size. There was no history of breast malignancy in the family or had a significant past medical or surgical history. On examination, a huge hard mobile lump of 12 cm × 10 cm was seen occupying the entire right breast. There was no clinical evidence of skin and chest wall infiltration. Axillary lymph nodes were palpable. Other breast and axilla were normal. Mammography revealed a large macrolobulated lesion in the right breast. Trucut biopsy from the lump revealed trabeculae and nests of plump cells with granular pink cytoplasm and round nuclei, possibly apocrine carcinoma. Hence, modified radical mastectomy was done.
The specimen showed a large lobulated tumor with tan surface of 10.5 cm × 9.5 cm × 5.5 cm Figure 1. Nipple, areola, and skin were grossly looking normal. Microscopically, the tumor cells were plump, arranged in lobules, and nests with focal tubule formation. The cells had abundant eosinophilic granular cytoplasm, large round nuclei, and prominent nucleoli Figure 2. Lymphovascular invasion was absent. Fifteen lymph nodes isolated from the axilla did not show metastasis. With the differentials of apocrine carcinoma and oncocytic carcinoma, panel of immunohistochemical markers inclusive of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor (Her2), androgen receptor (AR), epithelial membrane antigen (EMA), and gross cystic disease fluid protein (GCDFP-15) were done. The tumor cells were strongly reactive to AR Figure 3a, Her2 Figure 3b, EMA Figure 3c, and GCDFP-15 Figure 3d with the proliferation index of 26%. ER and PR were negative. Electron microscopy of the tumor showed abundant cytoplasm containing membrane-bound electron-dense granules Figure 4a, numerous dilated and swollen mitochondria Figure 4b, and multiple secretory vesicles. With the above histomorphological, immunohistochemical, and ultrastructural findings, a diagnosis of apocrine carcinoma was rendered.
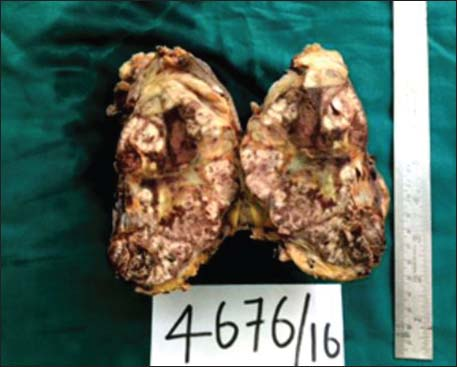
- Mastectomy specimen showing huge lobulated tumor occupying almost the entire breast parenchyma
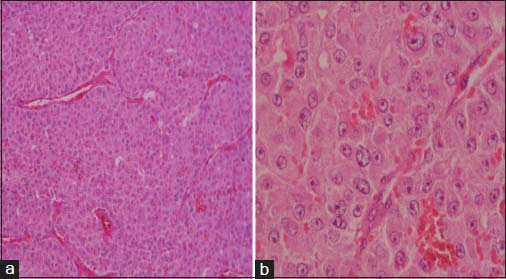
- (a) Histology showing tumor cells in nests with abundant eosinophilic cytoplasm (H and E ×100). (b) High power showing large nuclei with prominent nucleoli (H and E ×400)
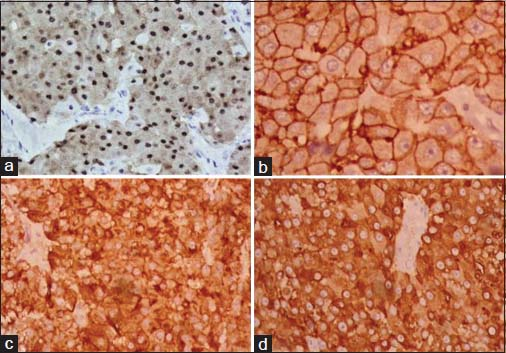
- The tumor cells show strong positivity to androgen receptor (a), human epidermal growth factor receptor 2 (b), epithelial membrane antigen (c), and gross cystic disease fluid protein-15 (d) (×400)
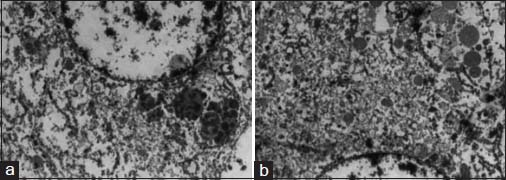
- Electron microscopy images show membrane-bound electron-dense granules (a) secretory vesicles and mitochondria (b)
Discussion
Apocrine carcinoma of the breast, an uncommon malignant neoplasm, accounts for about 0.5%–4% of invasive breast malignancies, characterized by extensive apocrine differentiation (in at least 90% of tumor cells).1,2 Focal apocrine differentiation is a common feature of invasive carcinoma, no special type as well as in lobular, tubular, micropapillary, and medullary carcinomas. It can be seen as well in lobular carcinoma in situ and ductal carcinoma in situ.3 The incidence of apocrine carcinoma varies considerably between studies ranging from 0.4% to 15%. This could be due to the lack of definite criteria for the diagnosis of apocrine carcinoma. Japaze et al. proposed that the diagnostic histologic criteria, in 2005, consist of (1) apocrine differentiation in at least 75% of the cells, (2) large cells with eosinophilic granular cytoplasm, (3) sharply defined cell borders, (4) nucleus-to-cytoplasmic ratio of 1:2 or more, and (5) pleomorphic vesicular nucleus. The minor criteria include prominent nucleoli in >50% of fields and apical snouts into luminal spaces.4
Clinically, apocrine carcinoma presents as a breast lump of any size with no predilection for quadrant or site. It usually occurs in elderly individuals and shows mammographic features similar to other invasive breast malignancies.1 Macroscopically, the tumor is lobulated, tan to brown with bulging cut surface.
Microscopically, the tumor shows a similar architectural growth pattern as invasive carcinoma, no special type with the difference in cytoplasmic characteristics. The cells show enlarged nuclei with prominent nucleoli and apical snouts. Based on the cytoplasmic characteristics, the cells are of two types. Type A cells are the one with abundant granular eosinophilic cytoplasm and show diastase-resistant periodic acid–Schiff positivity, whereas type B cells will have abundant foamy cytoplasm and may show intracytoplasmic lipid.1,2,5 Apocrine carcinoma may mimic oncocytic carcinoma or granular cell tumor, if type A cells predominate (as in our case). In such instances, it can be distinguished using a panel of immunohistochemical markers, ER, PR, Her2, AR, and GCDFP-15 as well with ultrastructural characteristics. Apocrine carcinoma shows ER, PR negativity, AR positivity, overexpression of Her2, and GCDFP-15. Hormonal profile of our case is similar as described in the literature. AR expression in apocrine carcinoma has high specificity although lacks sensitivity.1,6 Oncocytic carcinoma shows ER and PR positivity and is negative to AR Table 1.
|
Immunohistochemical markers |
Apocrine carcinoma |
Oncocytic carcinoma |
|---|---|---|
|
ER |
- |
+ |
|
PR |
- |
+ |
|
Her2 |
+ |
-/+ |
|
AR |
+ |
- |
|
GCDFP-15 |
+ |
- |
|
EMA |
+ |
- |
|
Ultrastructural features |
Electron-dense granules, abundant Golgi bodies mitochondria with incomplete cristae, and perinuclear condensation |
Numerous mitochondria occupying >60% of the cytoplasm |
+: Positive; -: Negative; ER - Estrogen receptor; PR - Progesterone receptor; Her2 - Human epidermal growth factor receptor; AR - Androgen receptor; GCDFP-15 - Gross cystic disease fluid protein; EMA - Epithelial membrane antigen
Ultrastructural examination of apocrine carcinoma shows cytoplasm rich in organelles, mitochondria with incomplete cristae and perinuclear condensation, abundant Golgi apparatus, electron-dense granules, and empty vesicles. Oncocytic carcinoma cells show abundant dispersed mitochondria, occupying >60% of the cytoplasm.7
Molecular apocrine breast cancer subtype was described by Farmer et al. by microarray analysis, characterized by AR expression and AR pathway activation.8 Naderi and Hughes-Davies et al. showed a crosstalk between AR and Her2, with FoxA1 activity as well as extracellular signal-regulated kinase (ERK) pathway, and found prolactin-induced protein (or GCDFP-15) as the actively regulated gene by the AR/ERK feedback loop in apocrine tumor cells. This explains the consistent expression of AR, GCDFP-15, and Her2 in apocrine carcinomas.9,10
Apocrine carcinoma of breast behaves like invasive carcinoma, no special type, when matched for grade and stage.1 Our case in spite of being huge (pT3) does not show lymphovascular emboli or lymph nodal metastasis which implies better prognosis. As these tumors express AR and Her2, the tumor shows a potential response to the administration of androgen analogs in addition to targeted therapy with Herceptin and adjuvant chemotherapy.9,10
Conclusion
Although apocrine carcinoma does not have specific predictive and prognostic factors and behaves like invasive carcinoma, no special type when matched for grade and stage, it needs to be diagnosed as a different entity. This is because of the fact that they show unique hormonal profile showing AR positivity with potential response to androgen analogs and may help in understanding the pathogenesis and ascertain the clinical behavior.
Financial support and sponsorship
Nil.
Acknowledgment
The authors would like to thank Professor, Dr Amanjit Bal, Department of Pathology, Postgraduate Institute of Medical Education and Research for helping us in the electron microscopical ultrastructural study of tumor and for its images.
Conflicts of interest
There are no conflicts of interest.
References
- Carcinomas with apocrine differentiation In: Lakhani S R, Ellis I O, Schnitt S J, Tan P H, Vande Vijver MJ, eds. WHO Classification of Tumours of the Breast (4th ed.). Lyon: IARC; 2012. p. :53-4. editors.
- [Google Scholar]
- Apocrine carcinoma of the breast: A comprehensive review. Histol Histopathol. 2013;28:1393-409.
- [Google Scholar]
- Benign and malignant apocrine lesions of the breast. Expert Rev Anticancer Ther. 2012;12:215-21.
- [Google Scholar]
- ‘Pure’ invasive apocrine carcinoma of the breast: A new clinicopathological entity? Breast. 2005;14:3-10.
- [Google Scholar]
- Apocrine carcinoma as triple-negative breast cancer: Novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol. 2012;42:375-86.
- [Google Scholar]
- Androgen receptor in breast cancer: Expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205-12.
- [Google Scholar]
- Electron microscopic studies of primary apocrine carcinoma of the breast. Med Electron Microsc. 1994;27:113.
- [Google Scholar]
- Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660-71.
- [Google Scholar]
- A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia. 2008;10:542-8.
- [Google Scholar]
- Prolactin-induced protein mediates cell invasion and regulates integrin signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012;14:R111.
- [Google Scholar]







