Translate this page into:
Tumor motion in lung cancers: An overview of four-dimensional radiotherapy treatment of lung cancers
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Most modern radiotherapy centers have adopted contouring based treatment. Sparing of the normal structures has been made more achievable than ever before by use of technologies such as Intensity Modulated Radiotherapy (IMRT) and Image guided radiotherapy (IGRT). However, unlike, sites such as brain or head neck, thorax is a site in active motion, mostly contributed by patient's respiratory movement. 4 D radiotherapy, that addresses the issues of motion in thoracic tumours answers this critical question. The present article outlines the scope of need for 4 D radiotherapy and discusses the options available for 4 D treatments of cancer patients.
Keywords
Four-dimensional treatment
lung cancers
tumor motion
Introduction
Lung cancer is a leading cause of cancer mortality throughout the world.1 Nearly 25% of lung cancer patients are candidates for curative treatment which includes the use of surgery, chemotherapy and radiotherapy (RT) or combination of these modalities.2 Operable lung cancer can be managed with surgery or surgery followed by RT and chemotherapy.3 Patients with locally advanced inoperable disease may achieve long-term survival with curative radiation therapy combined with chemotherapy. Most modern RT centers have adopted contouring-based treatment. Sparing of the normal structures has been made more achievable than ever before by use of technologies such as intensity-modulated RT (IMRT). Advanced image guidance tools ensure that there is concurrence between the planned treatment and the delivered treatment. A recent advance in lung RT is stereotactic conformal RT. In the case of lung cancer, the advantages of high precision and shorter overall treatment duration with this technique have translated into improved control and survival rates as well, compared to conventional RT.4 However, lung motion remains a critical factor in all cases planned for RT to the lung tumor.5,6
Rationale of Four-Dimensional Treatment Strategies
Respiratory motions, in its simplest forms represent a sine wave Figure 1. Physiological respiratory motion of primary lung tumors may reduce the chances of obtaining an optimal local control rate after RT. Respiratory organ motion can cause severe geometrical distortion in free-breathing computed tomography (CT) scanning. Distortions along the axis of motion could either lengthen or shorten the target length depending on the complex interplay of the magnitude and speed of organ motion, gantry rotation speed and pitch, resulting in random variations in the target shape.5 In addition to shape distortion, the center of the imaged target can be displaced by as much as the amplitude of the motion. Tumor motion for lung lesions during a respiratory cycle is well recognized.6 This motion is variable depending on the patient respiratory physiology, tumor size, tumor location, and the immobilization device used.7 Treating using 4D technology can have several benefits which include reduction in planning target volume (PTV) size, ensuring the planned dose and delivered dose concurrence, reduction in normal tissue dose, and the possibility of further dose escalation in some cases.8
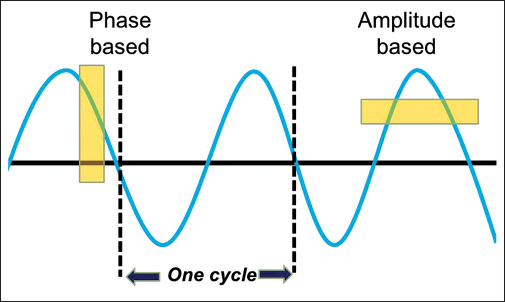
- The respiratory sine wave
Techniques of Four-Dimensional Scanning
Every 4D treatment delivery technique requires a way of acquiring 4D imaging information appropriate to the selected delivery technique. The 4D scanning methods or the acquisition of 4D imaging data can be classified under two categories: (i) CT scanning that does not require any type of hardware or software changes in the CT scanner and (ii) CT scanning that requires both or at least one of them. To the former type belongs the breath-hold CT scan (voluntary breath hold, active breathing control and combined inhale and exhale gross target volumes to get internal target volume) which does not need any kind of intervention to either the CT's hardware or CT's software. To the other group belongs the slow CT scan (4 s per slice in axial mode), prospective gated CT scan (images at only 1 phase with longer acquisition times that are 4 to 5 times longer than conventional scans), and 4D CT scan (three-dimensional [3D] scans at multiple phases), all of which require some kind of modification to the scanner's hardware or its software or both.9
In one of these methods, called retrospective 4D gated CT, at every position of interest along the patient's long axis, multiple images are obtained spanning the entire breathing cycle. Each image is tagged with the phase information of the breathing cycle it belongs to. Images are sorted retrospectively based on the breathing phase tags leading to many 3D CT sets, each corresponding to a particular breathing phase. Together, they constitute a 4D CT data set that covers the entire breathing cycle. One or more selective 3D CT data sets belonging to consecutive breathing phases can then be combined, averaged, and employed for various treatment techniques that account for respiratory motion.10 In this way, systematic errors can be reduced, and reliable target margins can be defined. This enables avoiding the risk of underdosing due to tumor motion. An added benefit is the reduction in the size of the planning target volume (PTV). Intuitively, this improves the therapeutic ratio by raising the dose to the tumor and decreasing the dose to the surrounding normal tissues.11 In addition, a 4D CT also enables to generate maximum intensity projection images. These images are a summation of the tumor position in all phases of the respiratory cycle.12
Techniques of Four-Dimensional Treatments
Several approaches are available for correcting for tumor motion at the time of treatment potentially leading to better conformality of dose to the target volume. These include synchronizing the beam-on/beam-off time with respiratory motion (gating), holding the patient in a particular phase of breath and treating in that breath-hold phase or targeting the tumor in all the phases of the respiratory cycle (tumor tracking).13,14,15
Gating technique
The gated RT technique is a noninvasive, video-based system that allows for imaging and treatment of lung, breast, and upper abdominal sites. Varian's (Varian, Palo Alto, CA, USA) real-time position management is a typical example of such gated delivery technique. It consists of an infrared tracking camera and a reflective marker that measures patient's respiratory pattern and range of motion and displays them as a waveform. The reflective marker block is placed on the patient's chest. This system can accommodate both breath-hold and free breathing protocols.16 The gating thresholds are set and these determine when the gating system turns the treatment beam on and off.17,18,19
With respiratory gating approaches, the patient continues to breathe normally. The radiation beam is turned on only within a specified portion of the patient's breathing cycle, which is commonly referred to as the “gate.” The position and width of the gate are determined by monitoring the patient's respiratory motion using an external respiration signal. The delivery of radiation during a limited portion of the breathing cycle can substantially reduce the duty cycle (the ratio of the gate width to the respiratory cycle period) and thus, increase the treatment time. The duty cycle is typically about 25%. Lesion motion and gating model stability, which can adversely impact the planned dose distribution, are also challenges for gating methods.
Duty cycle concept
Duty cycle is the ratio of beam-on time to the total treatment time (a measure of the treatment efficiency). For a nongated 3D conformal radiation therapy, the duty cycle would be 100% which reduces to about 30%–50% during a gated approach. For gated step-and-shoot IMRT treatment, the duty cycle would be lower than 30% due to the beam-off time needed for multileaf collimator (MLC) leaf motion.
Phase gating and amplitude gating
Amplitude-based gating allows automatic gating related to the absolute position of the marker block on the patient's thorax or abdomen, regardless of the phases in the patient's respiratory cycle Figure 1. Phase-based gating allows automatic gating of image acquisition and treatment delivery on the same phase of the patient's respiratory cycle.
Tracking techniques
Real-time tracking requires a method to move or shape the radiation beam relative to the moving target. For photon beams, there are three main ways to achieve this: (i) move the patient using the treatment couch; (ii) change the aperture of the collimator; and (iii) move the beam by physically repositioning the radiation source. Robotic couch-based motion tracking has been shown to be technically feasible for real-time compensation of intrafraction respiratory motion. However, continuous couch motion associated with real-time respiratory motion tracking has the practical issues of patient comfort, patient safety, and treatment tolerance.19
Alternatively, the beam can be effectively moved by changing the aperture of the collimator. The technical feasibility of this approach has been demonstrated for an MLC. However, there are several potential technical limitations to this approach. For example, the MLC motion required for target tracking superimposes on that required for intensity modulation, increasing the chances of exceeding the physical speed limitations of the MLC.20,21
A third approach is to physically reposition the radiation source to follow the tumor's changing position called as the real-time tracking RT. Available commercial systems allow irradiation of extracranial tumors that move due to respiration. One advantage is that patients can breathe normally during continuous treatment, enabling no reduction in the duty cycle.22 The primary concept in such systems is a correlation model between internal tumor position and external marker position. Synthetic X-ray images, commonly referred to as digitally reconstructed radiographs (DRRs), are generated from the treatment planning CT image by casting rays through the CT image using the known X-ray imaging system geometry to simulate the X-ray image formation process. The tumor position can be determined by aligning the positions of implanted fiducial markers in the DRRs with the marker locations in the X-ray images. Alternatively, direct tumor tracking can be performed by image registration of the tumor region in the DRRs to the corresponding region in the treatment X-ray images. The system uses external optical markers to provide a breathing signal. Three optical markers are attached to a snugly fitting vest the patient wears during treatment. The optical marker positions correspond to the chest wall position. Light-emitting diodes transmit light through optical fibers that terminate at the cylindrical optical marker. The optical markers are sequentially strobed and a stereo camera system, consisting of three linear charge-coupled device detector arrays, measures the 3D marker positions at regular frequency. The model adapts to gradual changes in target position and motion during treatment.22,23
Breath-hold techniques
Another set of approaches attempt to minimize the margin by delivering radiation when the tumor is at a relatively fixed and reproducible position.24 Breath-holding has long been used in diagnostic radiology to reduce the blurring of images. For radiation therapy, the goal is to attain the same breath-hold position maintained at the time of treatment planning CT scan during every treatment fraction.24,25
Breath-hold techniques provide noninvasive, internal immobilization of anatomies affected by respiratory motion. Such techniques have been widely used for stereotactic treatment in the lung and liver. Irradiation during deep inspiratory breath-hold is considered by some to have dosimetric advantages in terms of lung sparing through the inspiratory expansion of the healthy lung tissue.26,27 Breath-holding is physically demanding and uncomfortable, and breath-hold repeatability and patient compliance are challenging, especially for elderly patients or patients with compromised pulmonary capacity, common among patients with lung cancer or other pulmonary disease.26,27,28,29,30 Thus, breath-hold methods may not be applicable to a significant population of patients. On the other hand, end-expiration is considered to be more reliable by others because it is longer and more reproducible than end-inspiration. Table 1 gives a comprehensive comparison of the different 4D techniques that are available in the current clinical practice.
|
Gating |
Breath hold |
MIP |
Tracking |
|
|---|---|---|---|---|
|
4D CT required |
Yes |
No |
Yes |
Yes |
|
Patient compliance required |
++ |
+++ |
+ |
+ |
|
Worldwide experience till date |
+++ |
++ |
+++ |
+ |
|
Patient in free breathing during RT |
Yes |
No |
Yes |
Yes |
|
Duty cycle efficiency (%) |
30 |
30 |
>70 |
>70 |
|
Patient fitness/cooperation required for procedure |
Yes |
Yes |
No |
No |
+ minimal,++ strong,+++ very strong CT - Computed tomography; RT - Radiotherapy; MIP - Maximum intensity projection; 4D: Four-dimensional
Maximum intensity projection
One straightforward approach is to enlarge the clinical target volume to PTV, within which the target should move during the breathing cycle. The variation in target position associated with breathing can be determined by examining the range of target motion with fluoroscopy, slow CT scanning, or a 4D CT image study.31,32,33 The technique involves projection of maximum intensity of the tumor captured during all the phases of the respiratory cycle. The software then sums up the intensity of the tumor in all phases of respiration. This enables drawing an outline that encloses the tumor in all phases of respiration. The pitfall of this technique is the larger resulting volume as compared to breath-hold or gating techniques. Furthermore, this technique works well in lung lesions which are well surrounded by a rim of normal lung tissue. As such sites abutting the chest wall, mediastinum and the diaphragm are not the ideal sites for this technique.
Pitfalls of Four-Dimensional Techniques
Gating, without precisely knowing where the target is, may produce two kinds of errors (a) false positive in which beam is on at wrong target positions; (b) false-negative errors which mean that the beam is off at right target positions. The false negative reduces the treatment efficiency, while false positive causes underdose to the target and overdose to normal tissue. Further issues with 4D treatments include disparities between the external surrogate and internal tumor position.34 4D technique in general decreases the efficacy of RT delivery. As an example, RT beam spends around 80% of the respiration cycles switched off during gating while multiple breath-holds (with consequently increase overall treatment times) have to be used frequently.35 There is some controversy as to whether the selected phase of respiration should lie in end-inspiration or end-expiration.36 4D CT scan captures only a snapshot of respiration-induced tumor motion.37,38
Selection of Patients for Four-Dimensional Treatment Strategies
In general, 4D treatments are resource intensive at all levels. Besides escalating the cost of treatment, these techniques generally involve increased planning times and daily treatment times. As such, proper patient selection is a must for all 4D treatments. Factors such as patient's disease status, performance status, and tumor motion need to be taken into consideration. In an ideal workflow, all patients likely to benefit from 4D treatment process should be scanned with a 4D CT scan (including a free breathing scan). However, the decision of subjecting the patient to the 4D treatment process should be based on analysis of the 4D scan and other relevant factors. The recommended threshold of tumor motion where 4D treatment technique should be considered include >5 mm38 and >7.5 mm.39 Even after observing these criteria for 4D treatments, it is unlikely that any particular technique shall be ideal for every patient. There could be dosimetric implications of planning in inspiratory versus expiratory phase Table 2.
|
Inspiratory phase |
Expiratory phase |
|---|---|
|
Less time spent |
More time spent |
|
Lung expanded, V20 low |
Lung compressed, V20 higher |
|
Less density of lung |
More density of lung |
Conclusion
Respiratory organ motion can cause significant geometrical distortion in free-breathing CT scanning. Respiratory motion of primary lung tumors may reduce the chances of obtaining an optimal local control rate after RT. Modern day techniques of 4D scanning and treatment allow accounting for tumor motion and have significant potential to increase the therapeutic gain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11:1653-71.
- [Google Scholar]
- Impact of tumor control on survival in carcinoma of the lung treated with irradiation. Int J Radiat Oncol Biol Phys. 1986;12:539-47.
- [Google Scholar]
- High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: Technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2002;54:348-56.
- [Google Scholar]
- Four-dimensional computed tomography: Image formation and clinical protocol. Med Phys. 2005;32:874-89.
- [Google Scholar]
- Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:822-34.
- [Google Scholar]
- Factors influencing intrafractional target shifts in lung stereotactic body radiation therapy. Pract Radiat Oncol. 2014;4:e45-51. et al.
- [Google Scholar]
- Usefulness of four dimensional (4D) PET/CT imaging in the evaluation of thoracic lesions and in radiotherapy planning: Review of the literature. Lung Cancer. 2016;96:78-86.
- [Google Scholar]
- Evaluation of 4D CT acquisition methods designed to reduce artifacts. J Appl Clin Med Phys. 2015;16:4949. et al.
- [Google Scholar]
- Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys. 2008;70:1229-38. et al.
- [Google Scholar]
- Improvement of CT-based treatment-planning models of abdominal targets using static exhale imaging. Int J Radiat Oncol Biol Phys. 1998;41:939-43.
- [Google Scholar]
- Evaluation of the cone beam CT for internal target volume localization in lung stereotactic radiotherapy in comparison with 4D MIP images. Med Phys. 2013;40:111709.
- [Google Scholar]
- Detection of lung tumor movement in real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:304-10. et al.
- [Google Scholar]
- Evaluation of respiratory movement during gated radiotherapy using film and electronic portal imaging. Int J Radiat Oncol Biol Phys. 2002;52:522-31. et al.
- [Google Scholar]
- Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1187-95. et al.
- [Google Scholar]
- Interfractional anatomic variation in patients treated with respiration-gated radiotherapy. J Appl Clin Med Phys. 2005;6:19-32.
- [Google Scholar]
- A comparison of the respiratory signals acquired by different respiratory monitoring systems used in respiratory gated radiotherapy. Med Phys. 2010;37:6178-86. et al.
- [Google Scholar]
- Dosimetric and clinical benefits of respiratory-gated radiotherapy for lung and breast cancers: Results of the STIC 2003. Cancer Radiother. 2012;16:272-81.
- [Google Scholar]
- A concept for classification of optimal breathing pattern for use in radiotherapy tracking, based on respiratory tumor kinematics and minimum jerk analysis. Med Phys. 2016;43:3168-77.
- [Google Scholar]
- Lung stereotactic body radiotherapy with an MR-linac – Quantifying the impact of the magnetic field and real-time tumor tracking. Radiother Oncol. 2016;119:461-6. et al.
- [Google Scholar]
- Correlation and prediction uncertainties in the cyberknife synchrony respiratory tracking system. Med Phys. 2011;38:4036-44.
- [Google Scholar]
- A dosimetric comparison of real-time adaptive and non-adaptive radiotherapy: A multi-institutional study encompassing robotic, gimbaled, multileaf collimator and couch tracking. Radiother Oncol. 2016;119:159-65. et al.
- [Google Scholar]
- Quantitative assessment of irradiated lung volume and lung mass in breast cancer patients treated with tangential fields in combination with deep inspiration breath hold (DIBH) Strahlenther Onkol. 2010;186:157-62.
- [Google Scholar]
- Geometric uncertainties in voluntary deep inspiration breath hold radiotherapy for locally advanced lung cancer. Radiother Oncol. 2016;118:510-4. et al.
- [Google Scholar]
- Dosimetric evaluation of lung tumor immobilization using breath hold at deep inspiration. Int J Radiat Oncol Biol Phys. 2001;50:1091-8. et al.
- [Google Scholar]
- Deep inspiration breath-hold technique for lung tumors: The potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol Biol Phys. 1999;45:603-11.
- [Google Scholar]
- Clinical evaluation of X-ray voxel Monte Carlo versus pencil beam-based dose calculation in stereotactic body radiotherapy of lung cancer under normal and deep inspiration breath hold. Technol Cancer Res Treat. 2015;14:334-42.
- [Google Scholar]
- Clinical outcome of hypofractionated breath-hold image-guided SABR of primary lung tumors and lung metastases. Radiat Oncol. 2014;9:10. et al.
- [Google Scholar]
- Reduced lung dose during radiotherapy for thoracic esophageal carcinoma: VMAT combined with active breathing control for moderate DIBH. Radiat Oncol. 2013;8:291.
- [Google Scholar]
- Motion management for radical radiotherapy in non-small cell lung cancer. Clin Oncol (R Coll Radiol). 2014;26:67-80.
- [Google Scholar]
- Clinical implementation of intrafraction cone beam computed tomography imaging during lung tumor stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87:917-23. et al.
- [Google Scholar]
- Deep inspiration breath hold radiotherapy for locally advanced lung cancer: Comparison of different treatment techniques on target coverage, lung dose and treatment delivery time. Acta Oncol. 2013;52:1582-6. et al.
- [Google Scholar]
- Analysis of reproducibility of respiration-triggered gated radiotherapy for lung tumors. Radiother Oncol. 2008;87:59-64. et al.
- [Google Scholar]
- Evaluation of respiratory pattern during respiratory-gated radiotherapy. Australas Phys Eng Sci Med. 2014;37:731-42.
- [Google Scholar]
- Comparison of gating around end-expiration and end-inspiration in radiotherapy for lung cancer. Radiother Oncol. 2009;93:430-5.
- [Google Scholar]
- Respiratory gated beam delivery cannot facilitate margin reduction, unless combined with respiratory correlated image guidance. Radiother Oncol. 2008;86:61-8.
- [Google Scholar]
- Dosimetric impact of geometric errors due to respiratory motion prediction on dynamic multileaf collimator-based four-dimensional radiation delivery. Med Phys. 2005;32:1607-20.
- [Google Scholar]
- Prediction of 3D position of lung tumors from carina and diaphragm positions. Int J Radiat Oncol Biol Phys. 2004;72:S49. et al.
- [Google Scholar]







