Translate this page into:
Hypofractionation in postmastectomy breast irradiation. How safe are we in using standard tangentials?
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim: Hypofractionation in breast radiotherapy is gaining increasing relevance in routine clinical practice; however, gray areas remain on its safety. Majority of data regarding the same pertains to the treatment of the conserved breast. This study aimed to compare the use of standard wedge-based tangentials (two-dimensional [2D] TW) versus 3D conformal radiotherapy field in the field (3DCRT FIF) with the intent of evaluating if the latter would provide a dosimetric advantage.
Materials and Methods: Twenty-six postmastectomy patients were enrolled in this study. Comparative plans using 2D TW and 3DCRT FIF were generated to deliver 50 Gy in 25 fractions. Dosimetric parameters pertaining target dose, Homogeneity Index (HI), Conformity Index, and dose to normal structures were compared and analyzed. The parameters that achieved significance were evaluated using the hypofractionated plan.
Results: The 3DCRT FIF plan showed better planning target volume coverage, V95% (P < 0.001) and less cardiac dose (V30 and MD) as well as lung V20, V30, MD, and V5 for both lungs (P < 0.001). The dose to the left descending coronary artery (LAD) was also less with a trend toward significance (P = 0.07). The 3DCRT FIF plan also improved HI (P = 0.02). However, the high-dose volume V107% and radiation exposure were not higher. The significant parameters were evaluated in the hypofractionated schedule of 40 Gy/15 Fr. The MD to the heart was 8.96 Gy in FIF plan versus 20.16 Gy in TW plan. The average V20 to the ipsilateral lung was 37.8% versus 65.2%. The average dose to the contralateral breast was 50% less, i.e., 3.92 Gy versus 8.96 Gy.
Conclusion: The results of this study suggest that there would be a significant benefit of using 3DCRT FIF plans for patients being considered for hypofractionated radiotherapy in the postmastectomy setup.
Keywords
Breast radiotherapy
field in field three-dimensional conformal radiotherapy
hypofractionation
Introduction
Breast cancer is the second most common cancer in India and leading cancer in women worldwide. Radiotherapy is an integral part of its multimodal treatment with a proven survival advantage for patients undergoing breast-conserving surgery and those patients with high-risk pathological features following modified radical mastectomy.1,2
The past few decades have seen dramatic changes in the approach to radiotherapy delivery and technique as well as the concepts of fractionation. Hypofractionated schedules have become the standard in the United Kingdom and many centers in Canada and gaining popularity in other parts of the world including India. Four major randomized trials involving nearly 7000 patients have updated results to suggest that hypofractionated schedules can provide an equivalent survival benefit and local control to the earlier standard of care 50 Gy delivered in conventional 2 Gy per fractions over a period of 5 weeks.3,4 The most popular regimes that have been adopted are 42.5 Gy in 16 fractions as well as 40 Gy in 15 fractions. The START A, START B, and Ontario trials have >10 years of follow-up to suggest favorable cosmesis. Although the incidence of cardiac morbidity was not significantly different, this parameter was less well analyzed and documented. The fact that <15% of patients involved in these trials had undergone mastectomy compromises the extrapolation of safety to this category of patients. Regarding the efficacy of hypofractionation in the postmastectomy setup, a few prospective trials do show equivalence. However, there are insufficient mature data to suggest the same as regards the late toxicity of heart and lung.5 The extrapolation of the currently available data to the postmastectomy setup faces several unaddressed issues. Patients undergoing mastectomy usually have more aggressive disease necessitating cardiotoxic neoadjuvant or adjuvant chemotherapy that can contribute to cardiac morbidity. The postmastectomy planning target volume (PTV) is inclusive of the chest wall and will be associated with a larger volume of heart and lung in the high-dose frame.
The most common technique of radiotherapy delivery to the postmastectomy chest wall is two-dimensional (2D) TW-based beams. However, in the past few decades, better radiotherapy techniques have evolved. There is evidence to suggest that 3D conformal radiotherapy field in the field (3DCRT FIF), tangential (inverse planning) intensity modulated radiotherapy and multifield intensity modulated radiotherapy may have dosimetric advantages over conventional tangentials in terms of heart and lung sparing.6
The current study was carried out with the objective of evaluating the dosimetric benefit of considering 3DCRT FIF over standard 2D TW when using hypofractionation in the postmastectomy setup.
Materials and Methods
Twenty-six patients with locally advanced breast cancer planned for postmastectomy irradiation were considered for this study. Eleven patients (42%) had left-sided lesions and 20 patients (76%) required supraclavicular irradiation. The study was performed with approval of the institutional ethics committee.
Plan design
Patients were simulated in the supine position. The clinical target volume (CTV) and PTV were contoured based on the radiation therapy oncology group breast atlas guidelines7 and using eclipse treatment planning system 13.7. The organs at risk (OAR) contoured included heart, ipsilateral lung, and contralateral lung, both lungs, contralateral breast, humeral head, spinal cord, and LAD. The CTV was isotropically expanded by 1 cm in the chest wall region and with a 0.5 cm margin in the supraclavicular region to generate PTV. Two plans were generated for each patient using the Eclipse treatment planning system (Varian Medical System).
The PTV was prescribed to 50 Gy, and optimization constraint was to ensure V95% ≥ 47.5 Gy. The 2D TW based plan had two opposite half beams with appropriate wedge angles. The 3DCRT FIF plan had 3–5 subfields with multifield collimation to appropriately shield the heart and lungs and ensure Dmax of PTV did not exceed 52.5 Gy. The optimization parameters for planning about PTV were V52.5 Gy < 1%, V50 Gy > 95%, for heart V20 Gy < 15%, V30 Gy < 20%, for lungs ipsilateral lung V20 Gy < 20%, V30 Gy < 30%, to contralateral breast Dmean < 3 Gy and to LAD V20 Gy < 15%, V30 Gy < 20%. Conformity Index (CI) and homogeneity Index (HI) were generated to compare the qualities of the plans.
Treatment delivery was based on the optimized 3DCRT FIF plan and the data set generated for the 2D TW plan was used only for the dosimetric comparison and analysis.
Paired sample statistics and Student's “t”-test were used to evaluate planning goals/parameters. The parameters achieving statistical significance were then evaluated using the hypofractionated schedule of 40 Gy in 15 fractions at 2.6 Gy per fraction.
Results
The PTV and OAR parameters achieved and observed are represented in Table 1.
|
Structure |
Dose parameters |
Treatment plan |
Mean (%) |
P |
|---|---|---|---|---|
|
PTV heart |
V47.5 |
Plan A |
79±2.3 |
0.001 |
|
Plan B |
43.6±3.8 |
|||
|
V52.5 |
Plan A |
0.5±0.9 |
0.590 |
|
|
Plan B |
0.8±0.47 |
|||
|
Dmax |
Plan A |
55.8±0.46 |
0.291 |
|
|
Plan B |
56.5±0.46 |
|||
|
HI |
Plan A |
0.37±0.05 |
0.428 |
|
|
Plan B |
0.4±0.06 |
|||
|
CI |
Plan A |
1.08±0.07 |
0.538 |
|
|
Plan B |
1.15±0.09 |
|||
|
MU |
Plan A |
399.9±19.9 |
0.80 |
|
|
Plan B |
392.4±23.2 |
|||
|
Dmax |
Plan A |
45.2±2.3 |
0.101 |
|
|
Plan B |
49.2±0.5 |
|||
|
DMEAN |
Plan A |
6.45±1.02 |
0.001 |
|
|
Plan B |
22.5±2.1 |
|||
|
V30 |
Plan A |
8.39±1.9 |
0.001 |
|
|
Plan B |
45±4.9 |
|||
|
V35 |
Plan A |
7.7±1.8 |
0.001 |
|
|
Plan B |
39±4.9 |
|||
|
Lad mean |
Plan A |
14.9±3.8 |
0.07 |
|
|
Plan B |
42.9±14.6 |
|||
|
Ipsilateral lung |
DMEAN |
Plan A |
20.3±1.4 |
0.001 |
|
Plan B |
28.8±1.3 |
|||
|
V20 |
Plan A |
37.8±1.4 |
0.001 |
|
|
Plan B |
65.2±2.6 |
|||
|
V30 |
Plan A |
32.3±1.8 |
0.001 |
|
|
Plan B |
59.1±3.4 |
|||
|
Both lungs |
V5 |
Plan A |
27.2±1.4 |
0.001 |
|
Plan B |
41.9±1.8 |
|||
|
Contralateral breast |
DMEAN |
Plan A |
3.5±0.43 |
0.001 |
|
Plan B |
8.8±0.94 |
|||
|
Spinal cord |
DMAX |
Plan A |
34.7±3.9 |
0.63 |
|
Plan B |
31.9±4.4 |
|||
|
Ipsilateral humerus |
DMEAN |
Plan A |
19.9±3.4 |
0.86 |
|
Plan B |
20.7±3.4 |
PTV - Planning target volume; HI - Homogeneity index; CI - Conformity index; MU - Monitor units; Dmax - Maximum dose
Plan A represents the 3DCRT FIF plan and the values achieved for the 2D TW plan by plan B.
Plan comparison and structure parameters
The PTV parameters evaluated were Dmax (maximum dose), V47.5 (percentage of PTV receiving 95% of the prescribed dose) V52.5 indicating the dose hotspot area that received 107% of the prescribed dose, HI was calculated as (D2%–D98%)/D50%. CI as:
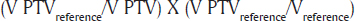
The parameters evaluated for OAR were Dmean, average dose delivered to an organ and Vx gray which was the percentage of the organ receiving ‘x’ Gy y or higher. We used paired sample, ‘t’-test, to compare normally distributed data between PLAN A and PLAN B.
Target coverage and dose parameters
The 3DCRT FIF plans showed statistically significant better coverage V47.5 (79 ± 2.3 vs. 43.6 ± 3.8, P < 0.001). Although the 3DCRT plan was more conformal (CI: 1.08 ± 0.7 vs. 1.15 ± 0.09) and homogenous (HI: 0.37 ± 0.5 vs. 0.4 ± 0.06), this did not attain statistically significant. There was notably no difference in monitor units involved in delivering treatment (399.9 ± 19.9 vs. 392.4 ± 0.807) in spite of the greater number of beams and collimation associated with the 3DCRT FIF.
Organs at risk – dose exposure
Heart and LAD
The 3DCRT FIF plan showed the maximum benefit for sparing the heart and coronary structures. A nearly threefold less dose compared to 2D TW plan which was statistically significant. The Dmean in 3DCRT FIF was 6.45 ± 1.02 versus 22.5 ± 2.1, P < 0.001 and V30 was 8.39 ± 1.9 versus 45 ± 4.9 for the 2D TW plan, P < 0.001. The dose received to LAD was nearly 50% less in the 3DCRT FIF showed a trend toward significance. The mean dose to LAD for the 3DCRT was 14.9 ± 3.8 versus 49.9 ± 14.6 for 2D TW plan (P = 0.07).
Ipsilateral lung and combined lung exposure
The 3DCRT plan showed better sparing of lungs in all the parameters evaluated, notably there was nearly 30%–40% reduction in dose exposure to the ipsilateral lung for the 3DCRT FIF plan V20 37.8 ± 1.4 versus 65.2 ± 2.6 in the 2D TW plan and this was statistically significant (P < 0.001). The 3DCRT FIF plan also provided better protection to the combined lung volumes V5 27.2 ± 1.4 versus 41.9 ± 1.8 (P < 0.001).
Other organs at risk
There was a significant reduction of dose exposure to the contralateral breast with 3DCRT plan 3.5 ± 0.43 versus 8.8 ± 0.94 (P < 0.001). There was, however, no difference in dose received by the ipsilateral humeral head or spinal cord.
Comparative evaluation with hypofractionation
The statistically significant parameter was evaluated in the hypofractionated schedule of 40 Gy in 15 fractions with biological equivalent dose conversions. The average equivalent dose received by the heart, i.e., mean dose to the heart was 8.96 Gy in 3DCRT versus 20.16 Gy in the 2D TW plan. The average V20 to ipsilateral lung is 37.8% in 3DCRT versus 65.2% in 2D TW plan. The average equivalent dose to the contralateral breast was 3.92 Gy in 3DCRT FIF plan versus 8.96 Gy in the 2D TW plan.
Discussion
The radiotherapeutic management of breast cancer has significantly evolved over the past few decades both in terms of wider availability, application of newer technology and a better understanding of biological response and toxicity to the breast tissue and other OAR associated with breast irradiation.
Hypofractionation in the adjuvant setup offers local control and adverse effects comparable to conventional fractionation. Four large randomized control trials have provided evidence to support its application following breast conservative surgery. Canadian, START A, START B, Royal Marsden Hospital and Gloucestershire oncology center.4,7 All these have conclusively shown equivalent roles of survival and local relapse as well as acceptable cosmesis with moderate hypofractionation. The advantage of using a shorter fractionation schedule includes better patient compliance, lower treatment cost-related to stay and travel, as well as better resource utilization. However, gray areas persist that prevent it from being the standard of care or being used routinely in the postmastectomy setup. Less than 15% of patients in START A, 8% in START B trial and none in the START pilot or Ontario trials had undergone modified radical mastectomy. The toxicities that have been addressed with the available follow up have mainly concentrated on cosmesis. In the postmastectomy set-up, there is the invariably larger amount of normal breast and lung volumes in the high dose cloud on account of including the chest wall as a part of the CTV. Majority of the patients undergoing postmastectomy radiation have the locally advanced disease and will receive chemotherapy that would contribute to anticipated cardiac and pulmonary toxicities.
In most centers in India as well as other parts of the world, postmastectomy irradiation is mainly delivered with two tangential wedge fields with additional supraclavicular fields when required. Although there are a number of prospective studies that provide insights into more sophisticated radiation techniques for treatment delivery to the intact breast, very few have addressed the issue in the postmastectomy setup.
We conducted this analytic study of dosimetric comparison of the treatment plans using 26 consecutive postmastectomy breast cancer patients to evaluate this issue. Several ongoing trials in breast radiotherapy are focusing on increasing conformity with intensity modifications with gated techniques.8 However, such facilities and options are not available to the majority of patients in India. In this study, we have focused on a practical alternative to standard 2D TW based plans that can provide better sparing to the heart and lungs and can be safely executed for patients considering hypofractionated treatment.
In our study, the 3DCRT plan showed a dosimetric advantage in terms of PTV coverage that was statistically significant. An earlier trial conducted by Cavey et al. evaluating 12 patients with these two treatment techniques also observed the same statistical benefit of PTV coverage (P < 0.001) as well as HI (P = 0.023).9 In our study, the 3DCRT FIF plans were more homogenous (HI) 0.37+/-0.05 versus 0.4+/-0.06. However, this was not statistically significant. The most significant results from our study were pertaining to the sparing of heart and lungs. The 3DCRT FIF plans clearly showed that the cardia could be better spared with nearly three times less exposure compared to the 2D TW technique, Dmean 6.45 ± 0.02 versus 22.5 ± 2.1(P < 0.001).
The relative proportionate risk of ischemic related cardiac events and mortality has been clearly established in several studies. In a population-based study considering 2168 women who underwent radiotherapy for breast cancer, Darby et al. observed a dosimetric correlation of mean dose received to the whole heart to the risk of major coronary events. They observed a risk of 7.4% per Gy (95% confidence interval 2.9–14.5, P < 0.001) with no apparent threshold.10 The increase incidence of ischemic events started at 5 years and continued to the third decade. Considering the same, the results of the current study would suggest a significant clinical impact.
Pulmonary toxicity in terms of dose received to the ipsilateral lung as well as both lungs was also significantly less V20, V30, and V5 (both lungs) P < 0.001. Besides pneumonitis, a higher incidence of second primary lung cancer has been associated with incremental exposure of the lung. Grantzau et al. in a population-based study involving 23,627 early breast cancer patients observed a linear association of second lung cancers with 8.5% per Gy (95% confidence interval = 3.1%–23.3%; P < 0.01).11,12
The other OAR that can be better spared with the 3DCRT FIF is the opposite breast. In our study, the Dmean in the 3DCRT FIF was nearly half of the value observed with the 2D TW plan 3.5 ± 0.43 versus 8.8 ± 0.94.
Conclusion
3DCRT FIF is dosimetrically more advantageous in terms of PTV coverage and sparing of the heart and lungs. It may not be practical to suggest this technique for all patients undergoing postmastectomy irradiation, as it is labor intensive for planning. However, it can be strongly recommended for patients undergoing hypofractionation and especially for left sided tumors when respiratory gating and image guidance is not an available option.
Financial support and sponsorship
Rajiv Gandhi University of Health Sciences research grant.
Conflicts of interest
There are no conflicts of interest.
References
- Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med. 2009;360:63-70.
- [Google Scholar]
- Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087-106.
- [Google Scholar]
- Hypofractionated adjuvant whole breast radiotherapy: Progress and prospects. Acta Oncol. 2010;49:1288-92.
- [Google Scholar]
- Postoperative radiotherapy following mastectomy for patients with left-sided breast cancer: A comparative dosimetric study. Med Dosim. 2015;40:190-4.
- [Google Scholar]
- Hypofractionation in post-mastectomy breast cancer patients: Seven-year follow-up. Med Oncol. 2012;29:2570-6.
- [Google Scholar]
- A comparative dosimetric study for treating left-sided breast cancer for small breast size using five different radiotherapy techniques: Conventional tangential field, filed-in-filed, tangential-IMRT, multi-beam IMRT and VMAT. Radiat Oncol. 2013;8:89.
- [Google Scholar]
- Postmastectomy hypo fractionated radiotherapy: May be a breakthrough in breast cancer management. Biomed J Sci Tech Res. 2017;1:4.
- [Google Scholar]
- Dosimetric comparison and evaluation of three radiotherapy techniques for use after modified radical mastectomy for locally advanced left-sided breast cancer. Sci Rep. 2015;5:12274.
- [Google Scholar]
- Dosimetric comparison of conventional and forward-planned intensity-modulated techniques for comprehensive locoregional irradiation of post-mastectomy left breast cancers. Med Dosim. 2005;30:107-16.
- [Google Scholar]
- Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987-98.
- [Google Scholar]
- Second primary cancers after adjuvant radiotherapy in early breast cancer patients: A national population based study under the Danish Breast Cancer Cooperative Group (DBCG) Radiother Oncol. 2013;106:42-9.
- [Google Scholar]
- Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol. 2014;111:366-73.
- [Google Scholar]







